SOCIÉTÉ INDUSTRIELLE DES PRODUITS PHARMACEUTIQUES
OUR MOTTO
DOUBLE-END, DOUBLE SAFETY
Since 1989, as the sole manufacturer of infusion solutions, our company has strengthened its core business by increasing production capacity from 4 to 12 million infusion bags per year. We have aligned our infrastructure and processes with current WHO standards for pharmaceutical manufacturing through consistent and sustained investments over the past decade.
Starting in 2024, with enhanced competitiveness of our products due to new equipment, we will cover 60% of the national demand for large volume infusion solutions.
Thanks to these investments and following inspections by the competent authorities of the Ministry of Public Health, as well as a site visit by the Minister of Health and his delegation, SIPP emerges in 2024 as a pharmaceutical manufacturing unit adhering to current Good Manufacturing Practices (GMP). This achievement led to the issuance of Certificate of Conformity No. D23 628/CBPD/MINSANTE/SG/DPLM/SDP/ SA on March 18, 2024.
SOME
FIGURES
millions
bags/year from 2024
%
local market
shares
distribution
centers
references

OUR
PRODUCTS
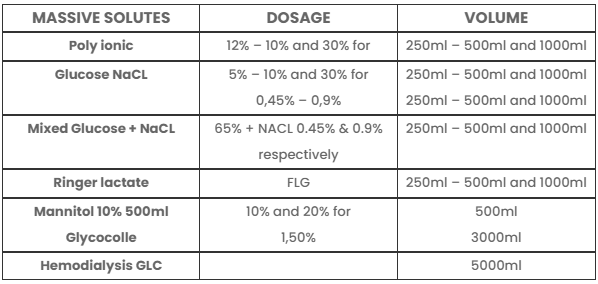

MASSIVE SOLUTES
DOSAGE
VOLUME
Poly ionic
12% – 10% and 30% for
250ml – 500ml and 1000ml
Glucose NaCL
0,45% – 0,9%
250ml – 500ml and 1000ml
Mixed Glucose + NaCL
65% + NACL 0.45% & 0.9% respectively
250ml – 500ml and 1000ml
Ringer lactate
FLG
250ml – 500ml and 1000ml
Mannitol 10% 500ml Glycocolle
1,50%
3000ml
Hemodialysis GLC
5000ml